
7505 Fannin St. Ste 610
Houston, TX 77054
ph: 713-467-2607
fda510k
Human Factor Engineering Validation
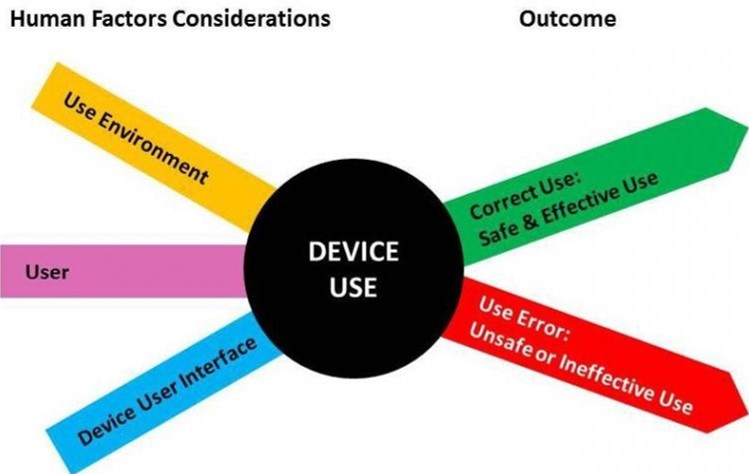
Regulatory Basis for HR
Medical Devices; cGMP Quality System Regulation Preamble to Final Rule 21 CFR Parts 808, 812, an 820
i.72. .." When designing a device, the manufacturer should conduct appropriate human factors studies, analyses, an tests from the early stages of the design proces until that point in development at which the interfaces with the medical profesional and the patient are fixed..."
i. 159. .."FDA emphassizes that any death, even if the manufacturer attributes it to user error, will be considere relevant by FDA an will have a high risk potentially associated with it. User error is still considered to be a nonconformity because human factors should have been conssiered during the design phase of the device."
- Preliminary Analyses and Evaluation
- Elimination or Reduction of Use Related Hazards
- Human Factors Validation Testing
- Test Participants
- Tasks and Use Scenarios
- Instructions for Use
- Participants Training
- Data Collection (Objective)
- Data Collection (Subjective)
- Analysis of Human Factors Validation Test Results
Copyright 2023 Mtech Group. All rights reserved.
7505 Fannin St. Ste 610
Houston, TX 77054
ph: 713-467-2607
fda510k